5/16/17 - Lab Report
Introduction: In NEW school, we are currently studying immunology, the reason for conducting this lab was to further understand how antibodies work in the body and get a hands on experience with our own blood types and what the antigens mean.
Purpose: How are antibodies used for blood typing?
Hypothesis: If my blood has antigens A and Rh present on the surface of my red blood cells, then my blood will precipitate or clump when exposed to the Antisera A and Anti-Rh factor because the anti- A and Rh serums will be considered as unknown pathogens to my antigens and they will swarm and attack them.
Materials:
- Water
- Bleach
- 1 Tub
- 1 Lancet
- 1 Blood typing slide
- Antisera A and B
- Anti-Rh factor
- Mixing sticks
- 1 Live Human/Victim
- 1 Pair Disposable gloves
- 1 Wet Wipe
Procedure:
- Create a data table to record all your data and observations.
- Prepare a sterile blood typing slide.
- Add one drop of synthetic anti-A to the A section.
- Add one drop of synthetic anti-B serum to the B section.
- Add one drop of synthetic anti-Rh serum to the Rh section.
- Clean finger that will be pricked with alcohol wipe, make sure that whoever is handling blood that isn’t theirs is wearing gloves.
- Twist and pull blue safety pin from lancet.
- Place lancet on the finger and push the needle into the skin.
- Squeeze one drop of blood into each serum.
- Use mixing sticks to mix the blood in with the serum, only the person that has been pricked should be handling their own blood. Discard each mixing stick after a single use to avoid contamination to your samples.
- After 30 seconds of stirring carefully examine the liquid in the wells.
- If there is no reaction and the liquid is clear or light pink with no particles or cloudiness formed, you should mark "No" in the appropriate box in the data table.
- If there is any reaction such as solid particles that have formed (they may be darker or lighter than the original liquid) or a cloudy appearance then you should mark "Yes" in the appropriate box.
- Look very closely at the wells and only do one well at a time. Be sure to stir at least 30 seconds with the plastic stick.
Data:
|
Sample Number
|
Reaction With Anti -A y/n
|
Reaction with Anti-B y/n
|
Reaction with anti-Rh (y/n)
|
Determined blood type
| |
1
|
1
|
yes
|
no
|
yes
|
A+
| |
2
| ||||||
3
| ||||||
4
|
Conclusion:
In conclusion, the above experiment was a success and the students were easily able to see and test their own blood. The hypothesis was correct, my blood has the A and Rh antigens, meaning it will react to the serum. My blood did not react to the B, meaning that my blood type is A+. There weren’t any errors to my knowledge during the lab, it worked smoothly with no bumps in the road. There is always a possibility of error, especially in a classroom. If the NEW students had access to more advanced tools and a better environment, then the lab results might be more credible. Since there weren’t any errors, there isn’t really anything I would do differently in the lab. Maybe have a better camera to take more detailed pictures of the reactions.
5/18/17 - Vaccine Videos
To help NEW School students to better understand vaccines and the different opinions people have on them, there was an assignment to watch three videos and answer questions about each one to really get an idea of vaccines.
Video 1:
Video 2:
Video 3:
5/16/17 - Deeper Learning Dive
To better understand immunology, I selected a video that will help me in the areas that I am most confused with for this unit. I've been having some difficulty understanding antibodies and antigens so I selected this video by Nature Video.
I believe that this video is credible because the channel has a lot of subscribers, the video was posted a month ago, the narrator kept a professional tone throughout the video and the presentation during the video was very clean and organized.
From this video, I learned that the human body contains billions of antibodies for the purpose of fighting off pathogens. Each possible disease or bacteria has a specific antibody that can defeat it to help keep the human body healthy. Antibodies are prepared for almost any disease that the human body may come across due to all of the diversity in the genes of the antibodies.
This video helped me a lot as a learner to understand the topic mainly because of the visuals. Being a visual learner, videos with drawings and cartoons really help me to understand the topic. The narrator really helped as well because she talked at the perfect pace and was very easy to understand.
5/10/17 - Specific Immune System
Neto gave a lecture on the specific immune system and what happens in the body when a pathogen enters. This was an opportunity to further practice sketchnotes to prepare us for what will happen in college.
5/4/17 - Innate Immunity
- the immune system protects body from proteins, virus, bacteria, parasites, fungi which could harm the body (pathogens)
- First line of defense: keep them out
- skin
- oils on skin
- mucus membranes
- stomach acid
- Both first and second line are non specific immunity/innate
- Second line: what happens once the pathogens are inside the body
- inflammatory response like blood flowing and puss to fight the infection
- phagocytes, a cell that can eat up pathogens. Phagocytes have receptors/censors for things that should not be in the body
- Phagocyte will wrap around the pathogen and the pathogen will be engulfed inside
- Once engulfed, it is called a phagozome, a vesicle that contains the foreign particle
- Lygozymes attach to the phagozome and dump the contents into the bacteria
- phagocyte will take some subsets of the molecules and proteins
- Proteins = sequences of amino acids
- A protein that's broken up a lot, or a short sequence of amino acids is peptide chain
- phagocyte will take peptide chain and attach it to other proteins called major histocompatibility complex type 2 (MHC 2 protein)
- Now specific reactions come
5/2/17 - What are viruses? How do they work?
- A virus is a microscopic complex collection of organic matter that has the ability to self-replicate

Viral Tag Simulation - A virus has a shell made of protein that contains DNA or RNA and enzymes for replicating and manipulating their genetic materials
- Viruses are strands of DNA or RNA which is the virus' genome and helps replicate itself by infecting other cells
- Viruses love to infect healthy cells and convert them to virus producing robots
- To infect, the virus uses an outer protein coat covered in small receptors which allow it to bind and join to the membrane of the cell
- Once in the cell, the virus will use arsenal to interfere with the cell's protein manufacturing systems and DNA replication mechanism. Then the cell will replicate it's own DNA and manufacture proteins
- Soon the virus will replicate more and more, taking over other cells
- Some viruses are good, they can help strengthen disease resistance in crops and possibly created the first cell nucleus by infecting a bacteria
4/28/17 - Vaccine Presentations
Polio:
- Virus that affected younger aged people
- Created in 1900's
- 9 ingredients, 6 are benign, 3 toxic in high quantities
- Benign: vero cells, M 199, calf bovine serum, phenoxyethanol, neomycin, Eagle MEM, modified
- medium
- Toxic (in high quantities): Anything can be toxic in high quantities, formaldyhyde and stretomycin and polymyixin B are the 3 most toxic in polio.
DTaP:
- Used to treat tetnis, pertustis
- 15 ingredients
- Most are toxic in large quantities
- 5 benign, rest toxic
- There are a lot of extra ingredients that there isn't a lot of information on
- Ingredients with limited info; fenton medium containing bovine extract, modified latham medium derived from bovine caesin, and modified latham
MMR 2:
- treats measels, mumps and ramela
- 13 ingredients, none benign or toxic
- in high quantities, vitamins, amino acids and glutamate can be toxic
- Chick embryo cell culture and WI-38 human diploid lung fibrolass had limited information
HIB:
- treats influenza (flu)
- 12 ingredients
- non toxic: sodium chloride, sucrose, saline, lactose
- toxic: if allergic to milk-based proteins, modified mueller and miller medium can be dangerous
- There was limited information on synthetic mediums and amorphous aluminum hydroxyphosphate sulfate
Hepatitis B:
- treats hepatitis B
- First vaccine: 5 ingredients
- Second vaccine: 9 ingredients
- Non toxic: phosphate buffer, yeast protein, mineral salts, dextrose(sugar), soy peptone
- Toxic (in high quantities): formaldehyde (most dangerous when inhaled), sodium hyrdogen phosphate dihydrate, disodium phosphate dihydrate, aluminum hydroxide (combined with kidney failure), sodium chloride, potassium aluminum sulfate, amorphus aluminum hyroxyphosphate sulfate, amino acids
- There was limited information on mineral salts, sodium dihydrogen phosphate dihydrate, amorphous aluminum hydroxyphosphate sulfate
Hepatitis A:
- 16 ingredients
- benign: formalin, MRC-5 human diploid cells, aluminum hydroxide
- toxic (in high quantities): sodium chloride, sodium borate, aminoglycoside antibiotics, neomycin
- limited information on amorphous aluminum hydroxyphosphate sulfate
Varicella/Chicken Pox/ Varivax:
- 15 ingredients
- most everything is toxic in certain amounts
- non toxic: sucrose, hydrolized gelatin
- toxic: urea, neomycin, monosodius L-glutamate, sodium phosphate diabasic, potassium phosphate monobasic, potassium chloride, EDTA
- Limited information on human embryonic lung cell cultures, guinea pig cell cultures, human diploid cell cultures (WI-38), human diploid cell cultures (MRC -5)
HPV:
Questions:
- Who can have these vaccines based on alergies?
- Why are there unresearched ingredients?
- How many people experience undesired effects?
- How many grams is considered toxic?
- Is it more toxic for a younger kid? An elder?
Natural Gas with Scott
- Fracking: Hydrolic fracturing when they drill pumps into the ground, put water in at high pressure so it breaks rocks
- Cons: 9% of methane is leaked from the process of fracking due to water pressure
- Methane is potent and traps heat more than carbon dioxide
- After 20 year is 25 times more potent
- If it leaks into wells and water sources, people can light their water on fire
- People who live near oil and gas activities can be exposed to air toxins such as benzene (carcinogen) and emissions of smog-forming pollutants
- Environment: Methane is responsible for 11% of greenhouse gases, it's 86 times better at trapping heat in atmosphere as a greenhouse gas
- Fracking can cause Earth quakes by adding extra water that can shift the ground
 |
| Fracking Contamination |
Vocab:
- Hydraulic Fracturing - The process of extracting natural gas from mines via highly pressurized water
- Groundwater - The water found underground in the cracks of spaces in soil, sand and rock
- Contamination - The action of something becoming impure by polluting of poisoning
Oil with Nick:
- Oil is considered a fossil fuel: dead plants and animal matter that have been exposed to heat and pressure over the years
- Previously was small plants and animals like alga or zooplankton
- Diatom: plankton that was most often turned to oil
- Oil is collected by drilling into Earth and fill the outside with cement (in case of earthquake) and keep going until they find oil, they shoot it with sand or acid which causes the oil to come back up
- Refined: take out sand and acid from Earth
- Oil is found in rubber, aspirin, asphalt, perfumes, crayons and ink
- The US produces 9.4 million barrels of crude oil a day (394.8 million gallons produced every day)
- Barrel: 42 gallons
- Oil is a pollutant, when burned it releases CO2, CO, SO2 (greenhouse gases) which will heat up the planet and contribute to global warming
Coal with Colby:
- Coal is a dark combustible mineral substance consisting of carbonized vegetable matter used as fuel
- Coal is made through heat and pressure for millions of years, it takes 300 million years to form. The world was covered in swamps during the Carbonifere we got our coal from
- Types of coal: peat, lignite, subbituminous, bituminous and anthracite
- Peat is plant matter decomposed underwater, there is no oxygen present to break down the plant matter
- Anthracite is the coal that takes the longest to form and is most efficient
- Coal is primary used for heat and electricity
- 2,400,00 tons of coal was mined in 2015
- 1.7 billion tons of CO2 came from coal plants in the United States
- China has the most coal out of every single country in the world
- Coal - A black or dark brown combustible mineral substance consisting of carbonized vegetable matter, used as fuel
- (CFC)chlorofluorocarbon - compounds of carbon, fluorine, chlorine and hydrogen used as refrigerants. Foam-blowing agents solvents, and, formerly as atmospheric ozone layer.
- Carboniferous Period - A time period that was 329.2 to 200 million years ago. During this time the world was covered in swamps
Alternative Energy with Kelly
- Fossil fuels form over hundreds of millions of years from decomposed organic matter
- Intense pressure, high heat and time create fossil fuels
- Renewable resource: A substance that can be replenished just as fast as it can be drawn out and used
- Fossil fuels are considered a limited resource because they're nonrenewable because they cannot be replenished in a human lifetime
- Energy efficiency: the corresponding amount of energy produced by a given amount of fuel
- US uses 11 billion tons of oil in fossil fuels per year
- 4 billion tons of oil every year
- We are expected to run out of most fossil fuels by 2088
- Clean energy sources: an energy that does not pollute the atmosphere when used
- Clean energy sources are solar, wind, plants, energy harvested from water, steam collected from Earth
Political Ramifications with Casey
- Most of the oil is in the middle eastern side of the hemisphere
- Oil is hard to prop off of in the war
- Natural gas is the only reason that America is dominant in the energy industry
- Natural gas means more money, energy dependence
- China doesn't export, it uses it's own coal
- Energy paradox, the energy industries make more money when gas prices are up, energy
- All of out renewable sources is not efficient, nuclear is more efficient but it's dying out because a lot of people are afraid of it
- California being an environmentalist is harming the economy
- Every single energy source has a flaw, either not efficient or it kills you
Vocab:
- Export: Taking out, shipping our natural gas over seas
- Fossils fuels: Hydrocarbons that were formed after heat and pressure during swamp lands
- Energy Dependence: being dependable for your own energy
Environmental Impacts with Audrey
- Coal leads to smog, acid rain, toxins int eh environment and air pollution
- 1/3 of america's carbon dioxide comes from coal-fired power plants
- Burned coal emits, mercury, arsenic and lead which can negatively effect your health
- Oil fuels cars, planes and homes and can also make plastic
- Burned oil emits carbon dioxide, sulfur dioxide, nitrogen oxide and lead
- Oil spill destroy the insulating ability of fur- bearing mammals and cause water repellency
- Gases can be methane, ethane and propane
| Coal Forming |
Vocab:
- Air pollution: a mixture of solid particles and gases int he air that can contaminate the atmosphere
- Global warming: the term used to describe a gradual increase int he average temperature of the Earth's atmosphere and its oceans, a change that is believed to be permanently changing the climate
- CO2 Emissions: the production and discharge of something, especially gas or radiation
- The main contributor of carbon dioxide emissions are traffic or being in the car for a long time
- Fossil fuels are extracted from the earth and are millions of years old
Casey's Demo
Casey lit methanol on fire first which was clean because there was a clear blue flame. Natural gas would be considered more orange. It's considered clean because it's blue due there not being a lot of carbon in there. Ethanol is drinking alcohol from plants with a corn base, this flame was super clean because it had a small, blue flame. Gasoline was completely orange and the flame was very high, meaning it's extremely energy dense with a lot or carbon. A candle is made of hydrocarbons, the same as gas and had a steady flame which means it's a mix of all three of the previous gases. Cellulose's flame went out quickly and let out a large amount of dirty emissions.
- As organisms die they decompose under layers of sand
- Different types form based on temp., time, organic matter and pressure conditions
- 3 types: Coal, oil, natural gas
- Coal: formed from ferns plants treed hardened due to heat and pressure
- Oil: small organisms, pressure caused complex organic matter to decompose
- Natural gas: same as oil but with more heat and pressure
- fossil fuels = most used energy source
- Can be used for many different things (plastic, cosmetics, transportation)
- Can be expensive
- A lot of CO2
- Looking for alternatives
3/15/17 - Graphing With Sheets
- Identify independent variable & dependent variable (independent = x axis, dependent = y axis)
- Independent = thing that we are changing
- Dependent = thing we are observing that changes
- Highlight information to create graph, customize as necessary
- Necessary: Title, Label x & y axis,
3/10/17 - Water Cycle Lab
Materials:
- 150ml Beaker
- 500ml of water
- 6ml of salt
- Large glass bowl
- Saran wrap
- Ice
Procedure:
- Heat up 500ml of water for three minutes in the microwave
- Add 6ml of salt to the warm water
- Pour into a glass bowl to create a simulated ocean
- Cover the bowl in saran wrap
- Place a handful of ice on top of saran wrap
3/8/17 - The Water Cycle
 |
| Fig. 1 NOAA National Weather Service. The Water Cycle (2012). |
The water cycle is very important, just like water. The water cycle affects the Earth's ecosystem greatly and also affects other cycles with different elements. The water cycle is extremely complex and involves water moving through different ecosystems and changing it's state. Some parts of the water cycle move quickly while others can take a lot longer, it all depends on the location and conditions of the water. The sun plays a huge role in the water cycle, it warms ocean water, causing it to evaporate and move to the atmosphere where it can then fall as rain or snow after it precipitates. Once the rain or snow falls back down, there are different options as to what it can do next. The possibilities include, evaporating again, flow over the surface, or sink back into the ground (prelocate).
When the water reaches the Earth's surface it can be taken up by plant roots, or can move along the surface as runoff when the soil can't absorb it. When water prelocates into the subsoil, it creates ground water which can be found in the pores between the sand or gravel. The water cycle for groundwater takes a lot longer because it takes longer for the water to flow through the pores until it gets to another source of water like a stream or lake.
 |
| Fig. 2 Open Stax College, Biology. Biogeochemical Cycles. |
The speed that water moves depends greatly on the state that the water is in.
3/6/17 - Ecology Vocab
- Ecology (noun) the study of how living things interact with one another and their environment (eco = environment, ology = study of something)
- Ecosystem (noun) all of the living and non-living things in an area and their interactions with each other (system = parts working together)
- Abiotic factor (noun) the part of the ecosystem that is not alive and has never been alive (ex: weather is part of ecosystem but not living part)
- Biotic factor (noun) the part of an ecosystem that is alive (ex: trees, insects, animals)
- Adaptation (noun) a characteristic that helps an organism survive in its environment (ex: cactus has an adaptation of being able to store water to help it survive)
- Biome (noun) a plant and animal community that covers a large part of Earth (aquatic biome = ocean that covers 80% of Earth)
- Detritivore (noun) organism (as an earthworm or fungus) that feeds on dead and decomposing organic matter
- Community (noun) a group of organisms living together in a certain area
- Consumers (noun) an organism that survives by eating producers or other consumers in the ecosystem. (ex: Koala eats eucalyptus)
- Carnivore (noun) organism that only eats meat (ex: lion, jaguar, etc)
- Deforestation (verb) the cutting down and clearing of forest land- will usually lead to increased soil erosion in the area
2/27/17 - Flipped Classroom Videos
DNA Splitting and Replication
- DNA is found in the nucleus of our cells
- There are four nitrogenous bases in DNA, Adenine, Cytosine, Guanine, and Thymine
- The DNA strands are anti parallel meaning they are parallel but flow in different directions
- There is a 3 prime and a 5 prime end, the direction flows from 3 prime to 5 prime
- You can only add a nucleotide to the 3 prime end
- The pairs are AT and CG
- Once it has been split in two, the top prong is the leading strand and starts with 3 prime
RNA and Protein Synthesis
Proteins are incredibly important to the human body, they provide sight, give us the ability to move, protect us from bacteria, and since they are enzymes, they are responsible for every reaction in the human body.
How proteins are synthesized (made):
- DNA in nucleus is split
- DNA split like zipper, the nitrogenous base, thymine is replaced by uracil. RNA will always have uracil.
- RNA leaves the nucleus
- Codon is 1 set of 3 nucleotide
- Codons = bumps in the zipper of DNA
- Codons = code each give instructions for the RNA
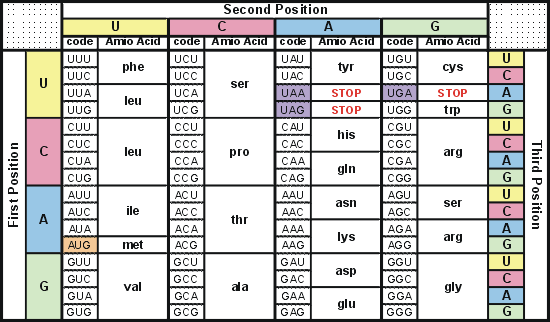
Ribosomes main purpose is to create proteins
Ribosome is made up of protein and rRNA is within the ribosome
Stamping occurs in the ribosomes (codon chart)
3 codons at a time until they hit a stop codon (ex. UAA)
Like a telegram, cuts it off
Mutations are dependent on reading the code
Substitution mutation (cause sickle cell amenia): It is substituting one of the nucleotides incorrectly. RNA is single stranded so it's really vulnerable
Ex: CTGGAG > CTGGGG

Insertion mutation (hunnington's disease): inserting a new
Ex: CTGGAG > CTGGTGGAG

Frameshift mutation: when one is missing and the rest get shifted over
Protein Synthesis and Mutation
Recap:
The hemoglobin was earlier taught when learning about cellular respiration, the hemoglobin will carry the oxygen cells. If the hemoglobin cannot deliver enough oxygen then our body will produce lactic acid because of it's protein mutations
Protein:
- Formed by long chains of amino acids (building blocks of life)
- DNA > RNA > Protein
- When DNA is mutated, it will cause mutated RNA, proteins, cells and tissues (type of system of order)
Protein Synthesis:
- Most basic biological process
- Cells build new protein
- Starts in nucleus
- RNA molecule travels to cell's cytoplasm
Mutation:
- An error in a DNA sequence, occurring when replicated
- Two types of mutations, natural or induced
- Natural: DNA copying
- Induced: chemicals in the environment, too much radiation, tobacco
- Missense vs Nonsense Mutations
- Missense: Mutation changes protein type
- Nonsense: mutation causes stop in translation
- Point mutations: changes one base in sequence
- substitution: changes one base in sequence
- deletion: remove one extra base in sequence
- insertion: add a base
- Frameshift Mutation
- caused by insertions or deletions in a number of nucleotides (building blocks in DNA) in a DNA sequence
- Sickle Mutation
- When hemoglobin is smaller and doesn't have enough space to carry oxygen
- Can cause episodes of pain, Lack of red blood cells, Infections
- Prevents cells from receiving oxygen > lactic acid
1/25/17 - DNA
Intro to DNA
DNA is found is chromosome in the nucleus of the cell.
Anything that is living has DNA. DNA is a molecule which is made up of different elements.
The structure of DNA is a double helix, ladder format that spins on itself. Inside are nitrogenous bases called pyrimidines and purines. Pyrimidines are single ones and purines are the double ones, you will never see purines matched up or pyrimidines matched up. The purine, adenine is always paired up with the pyrimidine, thymine. The pyrimidine, cytosine is always paired up with the purine, guanine.

DNA has three major functions,
- Gene Coding, the heredity that transfered from parent to offspring (eye color, hair color)
- Codes for proteins (amino acids) which are used for making enzymes, hormones, cellular repair and other molecules which are made from molecules which are coded from DNA
- DNA replication, which is vital for reproduction, restoration of cells and other vital situations
In the structure of DNA,
- The sequence of bases acts as information storage in the form of codes to build proteins
- Molecules are long to store more information
- The base pairing means that complementary strands of information can be replicated
- The double helix gives the molecule stability
- Hydrogen bonds allow for easy unzipping for copying and reading information
What is DNA?
The building blocks of DNA is a nucleotide
A nucleotide has 3 parts to it,
- A nitrogenous base. There is a lot of nitrogen in a nitrogenous base hence the name and there are two different types of nitrogenous bases. The first ones are Purines, which have two carbon chains. The more simple base is called a pyrimidine. There are four types in each DNA.
- Guanine (purine)
- Cytosine (prymidine)
- Thymine (pyrimidine)
- Adenine (purine)
- Sugar, in DNA its called the Deoxeribose sugar because it is missing an oxygen.
- Phosphate group
There is a 3 prime end in DNA and a 5 prime end in DNA which are the sides of carbon that is present. It flows from 3 prime to 5 prime. On the other side of the DNA it's going to go in the opposite direction. When building DNA (adding another nucleotide) it can only be added to the 3 prime end.


The back bone of every nucleotide is the same, deoxeribose sugar, phosphate and it repeats in that pattern. On the other side of the nucleotide it goes phosphate, deoxeribose sugar and repeats in that pattern instead. The inside of the nucleotide is where the purines and the pyrimidines. The Adenine is connected to hydrogen bonds on the other side.

All the atoms in a DNA is pulled together by a hydrogen bond which is very weak so if DNA is pulled in two different directions, it will unzip down the middle, but it will go back together if let go.
In the word deoxyribonucleic acid, deoxyribo comes from the deoxyribo sugar, the nucelic comes from the idea that the DNA is found inside the nucleus and acid comes from the phosphate group.
DNA is just like the ladder except is has been twisted like a helix and there are hydrogen bonds running down the DNA giving it a stable 3D shape.
1/12/17 - Gel Electrophoresis
Today in class we are working with DNA and forensics and the lab groups are working on creating our own gel electrophoresis boxes. We started this lab off by annotating an article on the procedure of making these boxes.

We started out with a box and then used two wires to run along opposite sides of the box to act as magnets for the DNA, RNA and proteins. We then cut some Styrofoam to be the comb of the gel box. The gel for our lab was made of agrose which we then poured into the box and put in the fridge to allow it to cool so that next class we can begin the experiment.
10/30/16 - Soil Lab (Revised)
In class we got visited by a guest teacher who taught us about soil. We completed a lab on the soil: Soil Lab
There were 7 tubs of different kinds of moist soil and then another 7 of different dry soils, the class walked around and felt the soil, described it, tested the moist soil's pH and tested the dry soils' water absorbancy then documented their observations on the document linked above.
A soil's pH determines the acidity or alkalinity of it to help farmers grow their crops. The pH is measured on a scale of one to ten with one being extremely acidic and 10 being extremely alkaline. Farmers can use a tool called a soil pH meter.
The optimum pH level for a plant is between 5.5 and 7. Every plant requires a different pH level so this is why farmers use this to their advantage so they can have the best soil for their crops. Here is just a small example of how each crop grows better with a different pH level: The optimum pH level for a plant is between 5.5 and 7. Every plant requires a different pH level so this is why farmers use this to their advantage so they can have the best soil for their crops.
10/21/16 - Iron Chef Lab
It has been one week since the beginning of the experiment and we have noticed some significant changes with the teeth in some of the beverages:
Coca Cola
Day 2:
Day 3:
Day 4:
Day 5:
At first, there was some buildup and plaque on the roots, that plaque overtime became more abundant and darker, then by day 5 the enamel is starting to stain.
Coffee
Day 2:
Day 3:
Day 4:
Day 5:
The coffee at first stains the tooth a little and then slowly, some plaque starts to buildup on the roots of the teeth and at the base of the enamel towards the end of the first five days.
Green Juice
Day 2:
Day 3:
Day 4:
Day 5:
Over the past five days, there has been some but not a lot of staining on the teeth, no notable plaque or buildup and no other signs of decay.
Water
Day 2:
Day 3:
Day 4:
Day 5:
There hasn't been much change over time to the teeth that were in water, which makes sense because there is no sugar or anything damaging to teeth in water. There doesn't seem to be any build up or plaque at all on the teeth and the enamel looks healthy.
Listerine
Day 2:
Day 3:
Day 4:
Day 5:
There is a lot of food coloring in the Listerine so immediately the roots of the tooth were stained a bright blue-green color. Towards the end of the first five days, the enamel is also beginning to get stained this color but it is not as bright. There doesn't seem to be any plaque or buildup.
10/7/16 - Cellular Respiration Lab
In class we completed a lab to explain the cellular respiration system: Where does all the good stuff go?
Mrs. Neto helped the class to understand this concept by using muffin making as an analogy.
The box of cake mix represented the sugar molecule, the eggs, oil and water mixed together represented the cytoplasm and the finished product (brownies) represented the ATP.
The Krebs Cycle was represented by students stirring the mix and distributing it into the baking pan to show how it is a continuous cycle of cells working together to create ATP (brownies) by getting all the ingredients together, mixing them, distributing them and then baking them for the final product of ATP.
The last step in making ATP for our bodies is the electron transport chain which can produce 24 - 32 ATP. This important process occurs in the inner mitochondrial membrane and requires oxygen to thoroughly work. There is an enzyme at work called the ATP Synthase which makes H+ and ADP+P1 this results in the creation of ATP.
9/29/16 - Iron Chef Lab (Day 2):
On the second day of the iron chef lab, our group had to complete more research and finish up any last observations or ideas. We finished it off with making a hypothesis:
If 15 human teeth are placed in a cup of coca cola, green juice, water, coffee and Listerine (3 for each beverage) for 14 days, then the tooth that was in the cup of coca cola will have the largest increase in weight because the coca cola contains the most corn syrup, which can cause a lot of plaque and buildup on teeth if not brushed frequently.
Our group will soon begin the experiment soon, we will then be monitoring it every day (except for weekends) and taking pictures to document how these beverages affect the teeth.
9/28/16 - Organs to Elements
After taking notes on the video Mrs. Neto prepared for the class that covered the system and functions of organs, cells and the elements inside them. Organs to Elements
I then created a series of quizlet slides on the vocabulary found in the video: Quizlet
9/27/16 - Soil Lab:
In class we got visited by a guest teacher who taught us about soil. We completed a lab on the soil: Soil Lab
There were 7 tubs of different kinds of moist soil and then another 7 of different dry soils, the class walked around and felt the soil, described it, tested the moist soil's pH and tested the dry soils' water absorbancy.
9/23/16 - Iron Chef Lab (Day 1):
We were given a box with the following ingredients inside
There is one bottle of Coca Cola and one bottle of Bolthouse Farms Green Goodness Juice
The nutrition facts for these ingredients are very different, the juice has fruits, vegetables and vitamins in it while the Coca Cola bottle contains high fructose corn syrup (HFCS), sugars and sodium.
Part of the Iron Chef Lab is to construct our own lab based on the ingredients given to us in our box, like the culinary show. We can add ingredients to our box in addition to what was given to us and the lab needs to be well thought out.
To start of our iron chef lab my group began by asking questions, observing and brainstorming on ideas,
- Is the Green Goodness Juice actually healthy?
- What materials are similar to that of teeth?
- Possible extra ingredients: lemon juice, mexican soda, water, fizzy water, plastic cups (small)
- Can we get enamel for the project? What else is in a tooth?
An idea for the experiment is to either get a real human tooth or a material similar to a tooth or enamel and drop one in a cup of every beverage in our box and leave it for a period of time to see how it affects the enamel and tooth.
Research:



No comments:
Post a Comment